Drug repurposing is often regarded as a cinderella child of new medicine discovery because ‘old drugs have expired patent exclusivities and the commercial opportunity is absent’. This is an erroneous view, because, in the first place, there are multiple ways to patent an old drug for a new use. And second, it is the differentiation of the new product relative to the old drug in its original indication, rather than a patent that underpins the necessary exclusivity.
But what if the old drug was never approved for marketing in the first place? Then, the necessity for differentiation is absent; such situations apply if the substrate for repurposing was abandoned midway along its development path, as is so often the case — sometimes for safety reasons, sometimes for efficacy reasons. The question then arises, is a patent necessary for the repurposed commercial development of an abandoned drug for a secondary use?
The answer, seemingly, is no. Let me quote an example.
Lonafarnib was originally discovered in the 1990s in an anticancer programme at Schering Plough, and given the drug research code SCH-66336. The development choice of cancer arose from the finding that mutant Ras proteins must be prenylated to exert their oncogenic effects, and that anticancer effects resulted with inhibitors of protein prenylation, specifically through the inhibition of protein farnesyltransferase. Industry researchers across the discovery-based pharmaceutical industry identified a number of compounds through either rational design, based on peptide- or isoprenoid-substrate characteristics, or through screening of in-house chemical libraries. However, as a class, protein farnesyltransferase inhibitors provided effects in cancer that were modest at best, with very few compounds showing anti-tumour activity. SCH-66336, although progressing far enough to earn the international non-proprietary name of lonafarnib, was ultimately abandoned for this indication.
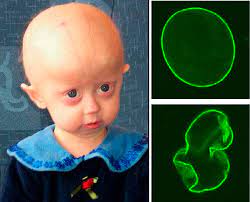
But what about other uses? Hutchinson-Gilford Progeria syndrome is a rare genetic disease that causes premature aging and death and has a debilitating effect on people’s lives. Patients with the syndrome experience accelerated cardiovascular disease from the buildup of defective progerin or progerin-like protein in cells. Most patients die before the age of 15 years from heart failure, heart attack or stroke.
Progeria results from a mutation in the LMNA gene, which produces protein Lamin A, partly through its farnesylation. It was proposed that protein farnesyltransferase inhibitors may ameliorate some of the disease phenotypes. Preclinical studies from 2006 found that treatment of progeria mice with lonafarnib could dramatically prevent the development of disease characteristics. These results prompted the launch of a phase II clinical trial examining the use of lonafarnib in progeria patients. This pilot was successful, resulting in a pivotal trial in 62 patients, in which the lifespan of those treated with lonafarnib increased by an average of three months through the first three years of treatment and by an average of 2.5 years through the maximum follow-up time of 11 years.
Lonafarnib was taken to both the US and European pharmaceutical regulators, and given approval under the brand namde Zokinvy in 2020 and 2022 respectively, with Orphan designation awarded in both jurisdictions. This gives the developing company Eiger Biopharmaceuticals market exclusivity for 7 and 10 years respectively, during which time no other product for Hutchinson-Gilford Progeria syndrome containing lonafarnib can be approved.
However, what about patent exclusivity? A quick review of the FDA Orange Book shows that two method of use patents will expire in October 2023 and July 2024. Patents therefore provide 3-4 years of commercial exclusivity in the US, whereas Orphan exclusivity doubles that. So, in essence, the commercial viability of Zokinvy depends on Orphan designation rather than patent exclusivity.
In a further twist, Eiger are also developing lonafarnib in combination with ritonavir for Hepatitis Delta virus (HDV), the most severe form of hepatitis, for which there are no approved treatments. HDV occurs only as a co-infection in individuals infected with Hepatitis B Virus, and leads to more severe liver disease, accelerated liver fibrosis, liver cancer, and liver failure. Approved treatments for Hepatitis B Virus have no impact on HDV. This development is in Phase III, and has also been awarded an Orphan designation in both US and EU. However, unlike the progeria development, the product for HDV is also protected by a composition of matter patent protecting the combination of lonafarnib and ritonavir that is due to expire in 2035.